So for my second youtube video i choose,i choose to pick the topic cells. The name of the video is A Tour of the Cell from the youtube channel bozemanbiology. The person carrying out the podcast was a teacher named Paul Anderson. I do hope you enjoy this summary and learn something valid 😀
As we know cells are small by the question is why? In the video it explains stating that cells are small for material to move in and out of the cell by diffusion,also for oxygen to go in and carbon dioxide to move out. When the surface’s area is increased and the volume is the same the distance for the materials and the gases to travel would be small. Cells are made up of different macromolecules and they are complex contrary to what you would think when you were younger. Until microscopes were invented we did’nt know the depth of the cells and the structures that lie within them. There are two types of microscopes which are the optical that uses light and electron which uses a number of magnets->scanning electron microscope and transmission electron microscope. In the electron microscope the specimens are dead. To help better understand he gived a example where the way a electron microscope operates is similar to that of putting a magnet close to a computer screen what happens is that the part of the electrons are changed increasing the magnification of the specimen.

ILLUSTRATIONS contrast the structure of an intestinal cell as known from light vs electron microscopy (left and right)
They are two types of cells……Prokaryotic cells and Eukaryotic cells. Prokaryotic cells have no nucleus and are very small whilst eukaryotic cells do have a nucleus. Bacteria and Arachaea belong to prokaryotic cells and plants animals and fungus among others belong to the eukaryotic cells. There are similarities between that all cells have whether prokaryotic or eukaryotic such as nucleic material,cell membranes,cytosol and ribosomes. The difference is that eukaryotic cells have organelles for example mitochondria.
Eukaryotes- animal cells. The video further went on to define and state the function of each organelle that can be found in the animal cell.

Illustration of the animal cell and its organelles.
Nucleolus are found in the nucleus. Here all the chromosomes put all of their genes to make ribosomes,therefore there is alot of proteins so it would stain darker. The nucleolus is the area where ribosomes are assemble in the nucleus. The genetic DNA also reside in the nucleus. There are also pores called nuclear pores in the nucleus that is used for translation where the material can move in and out.

Ribosomes have a small subunit on the bottom and a large subunit on the top. The function of ribosomes are to build proteins. Eukaryotic and Prokaryotic have different ribosomes.

Vesicle- This is a vacuum that moves material around.
Rough ER- ER stands for endoplasmic reticulum. This is a folded membrane that is located coming out from the nucleus,it has ribosomes that are on the outside of the membrane. “Factory behind a cell”,what is this? well as the messenger RNA comes through the rough ER it creates proteins and also produces proteins that are used in the cell.
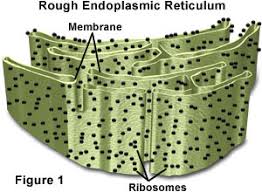
Illustration of the Rough ER
Golgi Body- Proteins are created in the endoplasmic reticulum and then moved to the Golgi Apparatus,here the proteins are modified by adding for example a carbohydrate and transported off. This is called the shipping part of the cell.
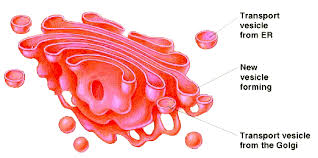
Illustration of the Golgi body
Cytoskeleton- The function of the cytoskeleton is to give the cell physical structure. A analogy was used here where a bridge is described example golden bridge,

where the two thick beams that are supporting it are the microtubules which give compressional support and the thin beams at the top on the left and right side going across are the microfilaments which give tensional support.
Smooth ER-From the name it can be told that there is no ribosomes instead the smooth ER produces lipids and cholesterol. They function in detoxification so the more you drink the greater amount of ER would build up in your body.
Mitochondria-This generates ATP (energy).It looks like a bacteria because it is believed that is becomes part of our cells through the endoplasmic theory where they become parts of the cell,produce ATP for the cell and dwells there. The evidence for this that they have there own DNA and reproduce through binary fission.

Illustration of the Mitochondria
Vacuole: This is found in plants and not exactly necessarily found in animals. It functions where it stored water for turgid pressure. Some protists have contractile vacuole. Animals used there vacuoles for endocytosis and exocytosis.

Illustration of the Vacoule
Cytosol: This can be described as the dissolved material in the cell which contains solutes.
Lyosome: Aka Suicide Sack. Digestive enzymes inside it which are contained by a membrane are able to go next to another vesicle that have material to breakdown and it would then go into the vesicle and break down the materials. Or when the vesicle is popped the digestive enzymes would kill the cells,this is called apoptosis.

Illustration of the lysosome
Lastly Centriole- Where ever this is sets up the location of the other organelles. When the cell divides the centriole would from the spindle pulling the chromosome aside.
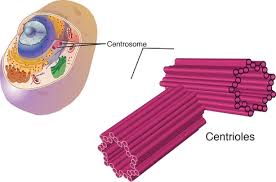
Illustration of the Centriole
Comments
My comments: I enjoy the way the person carrying out the video communicated with his audience it was free and a very easy mood. What aided in comprehending about cells was the analogies that was made and the easier names to remember the organelles and their functions for example lysosome aka suicide pack. In the end of the video a recap was done where questions were asked at the end,this is always a good way to know if you understand or took away anything from the topic. Furthermore though there were benefits i must say that there were some downfalls,the information given on each organelle was not done indepth,even the information given was somewhat lacking,for example the differences between the two type of cells prokaryotic and eukaryotic cells he just stated the organelles that both have and both,what about there size? the shape of the DNA?…prokaryotic has circular DNA whilst Eukaryote has linear DNA. What are the cells walls made up from? prokaryotic is made up of murein and eukaryotic cells are made up from plants-cellulose. Ribosomes number differ where prokaryotes are 70s and eukaryotes are 80s. Another downfall would be the lack of information given,for the role of the golgi body the cist phase and the transe phase was not mentioned,this occurs before the vesicles are shipped out. Only lysosomes were mentioned and not proteasome or peroxisomes. What i think need to be explained the most was in the area of the lysosome which initially gives me troubled,the lysosome are single celled organelles that contains the digestive enzymes protease etc They were initially formed by the Rough ER. Ribosomes would have synthesize the enzymes ,the rough ER would have carried out the modification(post translational modification) and send it off to the cyst phase this is where it would bind with the Golgi apparatus and they would be modified to go to their destination,the vesicles are accepted from the rough ER sorted and packed to be sent out. On the transe side their would be lysosomes budding off. The lysosme would fuse with a vesicle forming a secondary vesicle during phagocytosis. The cell engulfs some material from the outside forming a food vesicle and this would bind with the primary lysosome forming a secondary lysosome. The contents of the primary lysosome are released into the vesicle and digested ,phagocytosis takes place. I think they should of been explained more indepth like i just explained,due to the fact if a student whom knows nothing about cells watches this podcast they would not entirely know everything about cells. I would also like to add Apoptosis i did not know about this was interesting to learn about. Improvements such as being more indepth with the information and more questions could be done :).
I hope you enjoy and took what i said into account.
http://www.youtube.com/watch?v=1Z9pqST72is -link for the video used.
Websites for the pictures used:
http://www.animalport.com/animal-cells.html
http://www.cartage.org.lb/en/themes/sciences/zoology/animalphysiology/anatomy/animalcellstructure/GolgiApparatus/GolgiApparatus.htm
http://micro.magnet.fsu.edu/cells/plants/vacuole.html
http://www.howardmodels.com/fun-stuff/golden-gate-bridge/Golden-Gate-Bridge2.html
http://hyperphysics.phy-astr.gsu.edu/hbase/biology/cell.html